The purpose of the company’s clinical development program is to support the ongoing market introduction of the Ozilia® technology for chronic migraine in the EU and lay the foundation for regulatory market access in other markets such as the USA.
Our clinical development strategy is aimed to conduct studies of specific nature and shorter duration ourselves, and to encourage and facilitate other independent external investigator-initiated studies once such opportunities arise.
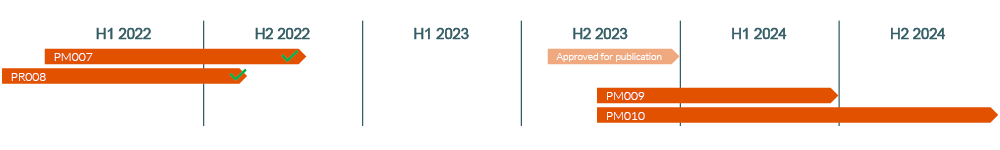
Ongoing & Planned Clinical Studies
The effect of Chordate’s treatment has been investigated in a number of studies over the years, where Chordate has often been the sponsor. These studies have been published in the articles below.
Tie-Qiang Li, Yanlu Wang, Rolf Hallin, Jan-Erik Juto. Resting-state fMRI study of acute migraine treatment with kinetic oscillation stimulation in nasal cavity. Neurolmage: Clinical 2016; 451-459
Jan-Erik Juto, MSc, MD, PhD; Rolf G. Hallin, MD, PhD. Kinetic Oscillation Stimulation as Treatment of Acute Migraine: A Randomized, Controlled Pilot Study. Wiley Periodicals, Inc. 2014
Fokkens et al. Rhinology Future Debates 2017 by EUFOREA: Novel treatments and surgical solutions in rhinology. Clinical Otolaryngology. 2018;1–10.
Ehnhage, A, Sahlstrand Johnsson P, et al Treatment of idiopathic rhinitis with kinetic oscillations – a multi- centre randomized controlled study. Acta Oto-laryngologica, 2016; 136(8):852-859
Juto JE, Axelsson M. Kinetic oscillation stimulation as treatment of non-allergic rhinitis: an RCT study. Acta Otolaryngol 2014; 134:506-12.
Markus Jerling MD, Iwona Cygankiewicz, Nabil Al-Tawil, Borje Darpo, Anders Ljungström, Wojciech Zareba. Effects of intranasal kinetic oscillation stimulation on heart rate Variability. Ann Noninvasive Electrocardiol. 2017;e12474.
Tie-Qiang Li, Yanlu Wang, Rolf Hallin, Jan-Erik Juto. Resting-state fMRI study of acute migraine treatment with kinetic oscillation stimulation in nasal cavity. NeuroImage: Clinical 12 (2016) 451–459
Sarin S. The role of the nervous system in rhinitis. J Allergy Clin Immunol 2006; 118:999-1016.
Safwan S. Jaradeh, MD; Timothy L. Smith, MD, MPH; Laura Torrico, MD; Thomas E. Prieto, PhD; Todd A. Loehrl, MD; Ronald J. Darling, MD; Robert J. Toohill, MD, FACS. Autonomic Nervous System Evaluation of Patients With Vasomotor Rhinitis. Laryngoscope 2000; 110:1828–1831
Hellings P.W., Klimek L., Cingi C., Agache I., Akdis C., Bachert C., Bousquet J., Demoly P., Gevaert P., Hox V., Hupin C., Kalogjera L., Manole F., Mösges R., Mullol J., Muluk N.B., Muraro A. , Papadopoulos N., Pawankar R. , Rondon C. , Rundenko M., Seys S.F., Toskala E., Van Gerven L., Zhang L., Zhang N., Fokkens W.J. Non-Allergic Rhinitis: Position paper of the European Academy of Allergology and Clinical Immunology. Allergy. 2017
K. Scadding, H. H. Kariyawasam, G. Scadding, R. Mirakian, R. J. Buckley, T. Dixon, S. R. Durham, S. Farooque, N. Jones, S. Leech, S. M. Nasser, R. Powell, G. Roberts, G. Rotiroti, A. Simpson, H. Smith, A. T. Clark, BSACI guideline for the diagnosis and management of allergic and non-allergic rhinitis (Revised Edition 2017; First edition 2007), Clin Exp Allergy. 2017;47:856–889.
Pete Smith, David Price, Richard Harvey, Andrew Simon Carney, Vicky Kritikos, Sinthia Z Bosnic-Anticevich, Louise Christian, Derek Skinner, Victoria Carter, Alice Marie Sybille Durieux. Medication-related costs of rhinitis in Australia: a NostraData cross sectional study of pharmacy purchases. Journal of Asthma and Allergy. 2017:10 153–161
For questions about Chordate’s share price or other investment-related topics, please see if the answer is here.
For questions about the medical effects of Ozilia®, please see if the answer is here.
To get in touch with Chordate regarding other questions, use the adjecent form. We do our best to respond as quickly as possible.
Please note that the MAR rules (Market Abuse Regulation) prohibits us from providing information to individual inquirers regarding matters of the company’s share, share price, economy and financials, commercial and scientific activities, and other information that potentially can alter the Market valuation of the traded share. We refer in general to the public information published by the company for such questions. Hence, emails with such questions will not be responded to individually.
Chordate Medical Holding AB (publ) is a medical technology company that has developed, patented and CE-marked Ozilia®, a neuromodulation and drug-free treatment technology for chronic migraine and chronic rhinitis. The treatment has clinically proven efficacy according to a recent study, and is marketed in selected markets in the EU and the Middle East. Chordate Medical is listed on Nasdaq First North Growth Market Stockholm (ticker: CMH).
The company’s Certified Adviser on Nasdaq First North Growth Market Stockholm is Bergs Securities AB.
Färögatan 33
SE-164 51 Kista
Sweden
For visits: floor B31
Delivery: Hanstavägen 31, 164 53 Kista